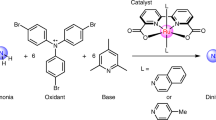
Abstract
DFT studies have been executed on a hypothetical Ru-triamidoamine complex to understand the possibility of synthesizing ammonia and hydrazine from the dinitrogen at normal temperature and pressure in heptane. In this present study, we utilized the \(\hbox {H}_{2}\) in the form of FLP-\(\hbox {H}_{2}\) complex and reacted with Ru-triamidoamine complex. We have added three \(\hbox {H}^{+}\) and \(\hbox {H}^{-}\) parts of FLP-\(\hbox {H}_{2}\) to the Ru complex in a stepwise manner as an alternate way to yield \(\hbox {NH}_{3}\)/\(\hbox {N}_{2}\hbox {H}_{4}\). The catalytic cycle for the formation of \(\hbox {NH}_{3}\) and \(\hbox {N}_{2}\hbox {H}_{4}\) were found to be energetically feasible. We have also observed some thermodynamically feasible six coordinate [M]-H intermediates.
Graphical Abstract
DFT studies were carried out on the possibility of synthesis of ammmonia and hydrazine from the dinitrogen using Ru-triamidoamine complex via FLP-\(\hbox {H}_{2}\) under normal experimental conditions. The calculated free energies revealed that the formation of all the intermediates and the transition states are thermodynamically viable. Furthermore, our calculations predicted that the formation of ammmonia is more feasible than hydrazine.












Similar content being viewed by others
References
Chatt J, Dilworth J R and Richards R L 1978 Recent advances in the chemistry of nitrogen fixation Chem. Rev. 78 589
Henderson R A, Leigh G J and Pickett C J 1983 The chemistry of nitrogen fixation and models for the reactions of nitrogenase Adv. Inorg. Chem. 27 197
Hidai M 1999 Chemical nitrogen fixation by molybdenum and tungsten complexes Coord. Chem. Rev. 185–186 99
Pickett C J and Talarmin J 1985 Electrosynthesis of ammonia Nature 317 652
Leigh G J 1992 Protonation of coordinated dinitrogen Acc. Chem. Res. 25 177
Hidai M and Mizobe Y 1995 Recent advances in the chemistry of dinitrogen complexes Chem. Rev. 95 1115
Kozak C M and Mountford P 2004 Revelations in dinitrogen activation and functionalization by metal complexes Angew. Chem. Int. Ed. 43 1186
Shilov A E 2003 Catalytic reduction of molecular nitrogen in solutions Russ. Chem. Bull. 52 2555
MacKay B A and Fryzuk M D 2004 Dinitrogen coordination chemistry: on the biomimetic borderlands Chem. Rev. 104 385
Hinrichsen S, Broda H, Gradert C, Söncksen L and Tuczek F 2012 Recent developments in synthetic nitrogen fixation Annu. Rep. Prog. Chem., Sect. A Inorg. Chem. 108 17
Tanabe Y and Nishibayashi Y 2013 Developing more sustainable processes for ammonia synthesis Coord. Chem. Rev. 257 2551
Jia H-P and Quadrelli E A 2014 Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen Chem. Soc. Rev. 43 547
Sivasankar C, Baskaran S, Tamizmani M and Ramakrishna K 2014 Lessons learned and lessons to be learned for developing homogeneous transition metal complexes catalyzed reduction of \(\text{ N }_{2}\) to ammonia J. Organomet. Chem. 752 44
Smil V 2001 In Enriching the Earth (Cambridge, MA: MIT Press)
Schlögl R 2003 Catalytic synthesis of ammonia—A ‘never-ending story’? Angew. Chem. Int. Ed. 42 2004
Bielawa H, Hinrichsen O, Birkner A and Muhler M 2001 The ammonia-synthesis catalyst of the next generation: Barium-promoted oxide-supported ruthenium Angew. Chem. Int. Ed. 40 1061
Burgess B K and Lowe D J 1996 Mechanism of molybdenum nitrogenase Chem. Rev. 96 2983
Dos Santos P C, Igarashi R, Lee H-I, Hoffman B M, Seefeldt L C and Dean D R 2005 Substrate interactions with the nitrogenase active site Acc. Chem. Res. 38 208
Burgess B K 1990 The iron-molybdenum cofactor of nitrogenase Chem. Rev. 90 1377
Howard J B and Rees D C 1996 Structural basis of biological nitrogen fixation Chem. Rev. 96 2965
Alien J D and Gawthorne J M 1986 Involvement of organic molybdenum compounds in the interaction between copper, molybdenum, and sulfur J. Inorg. Biochem. 27 95
Eady R R 1996 Structure-function relationships of alternative nitrogenases Chem. Rev. 96 3013
Hardy R W F and Gibson A H 1979 In A Treatise on Dinitrogen Fixation (New York: Wiley-Interscience)
Nishibayashi Y, Iwai S and Hidai M1998 Bimetallic system for nitrogen fixation: Ruthenium-assisted protonation of coordinated \(\text{ N }_{2}\) on tungsten with \(\text{ H }_{2}\) Science 279 540
Fryzuk M D, Love J B, Rettig S J and Young V G 1997 Transformation of coordinated dinitrogen by reaction with dihydrogen and primary silanes Science 275 1445
Pool J A, Lobkovsky E and Chirik P J 2004 Hydrogenation and cleavage of dinitrogen to ammonia with a zirconium complex Nature 427 527
Rodriguez M M, Bill E, Brennessel W W and Holland P L 2011 \(\text{ N }_{2}\) reduction and hydrogenation to ammonia by a molecular iron-potassium complex Science 334 780
Yandulov D V and Schrock R R 2002 Reduction of dinitrogen to ammonia at a well-protected reaction site in a molybdenum triamidoamine complex J. Am. Chem. Soc. 124 6252
Yandulov D V and Schrock R R 2003 Catalytic reduction of dintrogen to ammonia at a single molybdenum center Science 301 76
Yandulov D V, Schrock R R, Rheingold A L, Ceccarelli C and Davis W M 2003 Synthesis and reactions of molybdenum triamidoamine complexes containing hexaisopropylterphenyl substituents Inorg. Chem. 42 796
Ritleng V, Yandulov D V, Weare W W, Schrock R R, Hock A S and Davis W M 2004 Molybdenum triamidoamine complexes that contain hexa-tert-butylterphenyl, hexamethylterphenyl, or p-bromohexaisopropylterphenyl substituents. an examination of some catalyst variations for the catalytic reduction of dinitrogen J. Am. Chem. Soc. 126 6150
Yandulov D V and Schrock R R 2005 Studies relevant to catalytic reduction of dinitrogen to ammonia by molybdenum triamidoamine complexes Inorg. Chem. 44 1103
Schrock R R 2005 Catalytic reduction of dinitrogen to ammonia at a single molybdenum center Acc. Chem. Res. 38 955
Schrock R R 2005 Catalytic reduction of dinitrogen under mild conditions Chem. Comm. 2003 2389
Weare W W, Dai X, Byrnes M J, Chin J M, Schrock R R and Müller P 2006 Catalytic reduction of dinitrogen to ammonia at a single molybdenum center Proc. Natl. Acad. Sci. USA 103 17099
Weare W W, Schrock R R, Hock A S and Müller P 2006 Synthesis of molybdenum complexes that contain ‘hybrid’ triamidoamine ligands, [(hexaisopropylterphenyl-\(\text{ NCH }_{2}\text{ CH }_{2})_{2}\text{ NCH }_{2}\text{ CH }_{2}\text{ N-aryl }]_{3}\)-, and studies relevant to catalytic reduction of dinitrogen Inorg. Chem. 45 9185
Schrock R R 2008 Catalytic reduction of dinitrogen to ammonia by molybdenum: Theory versus experiment Angew. Chem. Int. Ed. 47 5512
Arashiba K, Miyake Y and Nishibayashi Y 2011 A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia Nat. Chem. 3 120
Tanaka H, Arashiba K, Kuriyama S, Sasada A, Nakajima K, Yoshizawa K and Nishibayashi Y 2014 Unique behaviour of dinitrogen-bridged dimolybdenum complexes bearing pincer ligand towards catalytic formation of ammonia Nat. Commun. 5 140
Arashiba K, Kinoshita E, Kuriyama S, Eizawa A, Nakajima K, Tanaka H, Yoshizawa K and Nishibayashi Y 2014 Catalytic formation of ammonia from molecular dinitrogen by use of dinitrogen-bridged dimolybdenum-dinitrogen complexes bearing PNP-pincer ligands: Remarkable effect of substituent at PNP-pincer ligand J. Am. Chem. Soc. 136 9719
Hölscher M, Prechtl M H G and Leitner W 2007 Can \([\text{ M(H) }_{2}(\text{ H }_{2})\)(PXP)] pincer complexes (M = Fe, Ru, Os; X = N, O, S) serve as catalyst lead structures for \(\text{ NH }_{3}\) synthesis from \(\text{ N }_{2 }\)and \(\text{ H }_{2}\)? Chem. A Eur. J. 13 6636
Hölscher M and Leitner W 2012 Heterolytic outer-sphere cleavage of \(\text{ H }_{2}\) for the reduction of \(\text{ N }_{2}\) in the coordination sphere of transition metals—A DFT study Angew. Chem. Int. Ed. 51 8225
Hölscher M and Leitner W 2006 DFT investigation of the potential of HM\((\text{ NHCH }_{2}\text{ CH }_{2})_{3}\)X catalysts (M = Mo, Ru, Os; X = N, P) for the reduction of \(\text{ N }_{2}\) to \(\text{ NH }_{3}\) by \(\text{ H }_{2}\) Eur. J. Inorg. Chem. 2006 4407
Truhlar D G 2009 Valence bond theory for chemical dynamics J. Comput. Chem. 28 73
Guha A K and Phukan A K 2011 Why vanadium complexes perform poorly in comparison to related molybdenum complexes in the catalytic reduction of dinitrogen to ammonia (schrock cycle): A theoretical study Inorg. Chem. 50 8826
Cao Z X, Zhou Z H, Wan H L and Zhang Q N 2005 Enzymatic and catalytic reduction of dinitrogen to ammonia: Density functional theory characterization of alternative molybdenum active sites Int. J. Quantum Chem. 103 344
Schenk S and Reiher M 2009 Ligands for dinitrogen fixation at schrock-type catalysts Inorg. Chem. 48 1638
Magistrato A, Robertazzi A and Carloni P 2007 Nitrogen fixation by a molybdenum catalyst mimicking the function of the nitrogenase enzyme: A critical evaluation of DFT and solvent effects J. Chem. Theory Comput. 3 1708
Khoroshun D V, Musaev D G and Morokuma K 2002 Sigma trans promotion effect in transition metal complexes: A manifestation of the composite nature of binding energy Mol. Phys. 100 523
Schenk S, Kirchner B and Reiher M 2009 A stable six-coordinate intermediate in ammonia-dinitrogen exchange at Schrock’s molybdenum catalyst Chem. A Eur. J. 15 5073
Schenk S, Guennic B-L, Kirchner B and Reiher M 2008 First-principles investigation of the Schrock mechanism of dinitrogen reduction employing the full \(\text{ HIPTN }_{3}\)N ligand Inorg. Chem. 47 3634
Studt F and Tuczek F 2005 Energetics and mechanism of a room-temperature catalytic process for ammonia synthesis (Schrock cycle): Comparison with biological nitrogen fixation Angew. Chem. Int. Ed. 44 5639
Hetterscheid D G H, Hanna B S and Schrock R R 2009 Molybdenum triamidoamine systems. Reactions involving dihydrogen relevant to catalytic reduction of dinitrogen Inorg. Chem. 48 8569
Balu P, Baskaran S, Kannappan V and Sivasankar C 2012 A possibility of functionalizing the dinitrogen in a Chatt complex by \(\text{ H }_{2}\): Density functional studies Polyhedron 31 676
Balu P, Baskaran S, Kannappan V and Sivasankar C 2012 Hydrogenation of dinitrogen to ammonia in [WF(\(\text{ PH }_{2}\)(\(\text{ CH }_{2})_{2}\text{ PH }_{2})_{2}\text{ N }_{2}\)] using \(\text{ H }_{2}\): Insights from DFT calculations New J. Chem. 36 562
Baskaran S and Sivasankar C 2013 Reduction of \(\text{ N }_{2}\) by \(\text{ H }_{2}\) to \(\text{ NH }_{3}\) and \(\text{ N }_{2}\text{ H }_{4}\) using [MoL] (L = triamidoamine) and organic co-catalysts: A theoretical approach J. Mol. Catal. A Chem. 370 140
Baskaran S and Sivasankar C 2014 Ammonia and hydrazine synthesis from [\(\text{ N }_{2}\)-W(\(\text{ NHCH }_{2}\text{ CH }_{2})_{3}\)N] and \([\text{ AH }]^{+}[\text{ BH }]^{-}\) using Sivasankar catalytic cycle: DFT studies Comput. Theor. Chem. 1027 73
Tamizmani M and Sivasankar C 2017 Protonation of coordinated dinitrogen using protons generated from molecular hydrogen Eur. J. Inorg. Chem. 2017 4239
Becke A D 1993 Density-functional thermochemistry. III. The role of exact exchange J. Chem. Phys. 98 5648
Becke A D 1988 Density-functional exchange-energy approximation with correct asymptotic behavior Phys. Rev. A 38 3098
Becke A D 1985 A new mixing of Hartree–Fock and local density-functional theories J. Chem. Phys. 98 1372
Hay P J and Wadt W R 1985 Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82 270
Wadt W R and Hay P J 1985 Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi J. Chem. Phys. 82 284
Hay P J and Wadt W R 1985 Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals J. Chem. Phys. 82 299
Dunning T-H and Hay P J 1977 In Methods of Electronic Structure Theory H F Schaefer III (Ed.) Vol. 3 (Boston: Springer)
Gordon M S 1980 The isomers of silacyclopropane Chem. Phys. Lett. 76 163
Hariharan P C and Pople J A 1973 The influence of polarization functions on molecular orbital hydrogenation energies Theor. Chim. Acta 28 213
Hariharan P C and Pople J A 1974 Accuracy of AHn equilibrium geometries by single determinant molecular orbital theory Mol. Phys. 27 209
Ditchfield R, Hehre W J and Pople J A 1971 Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules J. Chem. Phys. 54 724
Hehre W J, Ditchfield R and Pople J A 1972 Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules J. Chem. Phys. 56 2257
Mennucci B and Tomasi J 1997 Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries J. Chem. Phys. 106 5151
Cancès E, Mennucci B and Tomasi J 1997 A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics J. Chem. Phys. 107 3032
Cossi M, Barone V, Mennucci B and Tomasi J 1998 Ab initio study of ionic solutions by a polarizable continuum dielectric model Chem. Phys. Lett. 286 253
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Laham A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A Gaussian 03, Revision C.01. Gaussian, Inc.: Wallingford CT, 2003
Acknowledgements
C. S. thanks the Science and Engineering Research Board (EMR/2014/000623), New Delhi, India for the financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Christopher Jeyakumar, T., Baskaran, S. & Sivasankar, C. Possibility of reducing the coordinated dinitrogen into ammonia and hydrazine using [Ru-L] (L = triamidoamine) and FLP-H\(_{2}\): A DFT study. J Chem Sci 130, 57 (2018). https://doi.org/10.1007/s12039-018-1460-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1460-1